
Cleanroom Validation
Cleanroom Validation
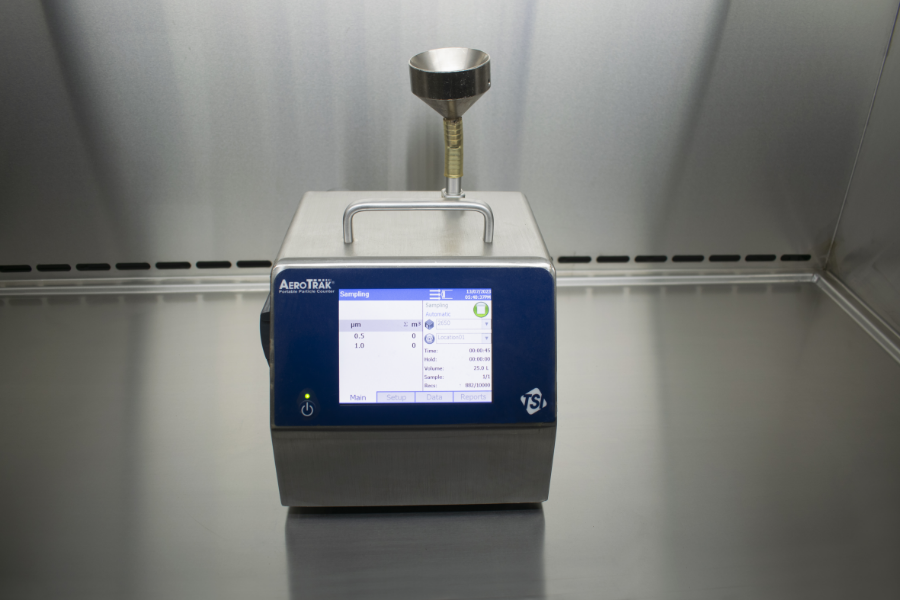


Cleanroom Validation
Cleanrooms play a critical role in numerous industries, from pharmaceuticals and biotechnology to electronics and aerospace. These controlled environments are designed to minimize airborne contamination and ensure product integrity, safety, and compliance with regulatory standards. However, maintaining the desired level of cleanliness is an ongoing challenge. That’s where Cleanroom Validation becomes paramount.
Cleanroom Validation is a comprehensive process that verifies the performance and effectiveness of your cleanroom facility. It involves a series of tests, measurements, and assessments to confirm that your cleanroom meets the stringent requirements set forth by international standards, such as ISO 14644-1. By performing regular cleanroom validation, you can:
Product Safety
Cleanroom validation ensures that the quality of your products remains uncompromised by verifying the cleanliness of the environment where they are manufactured, packaged, or stored. This minimizes the risk of contamination-related defects and protects your brand reputation.
Regulatory Compliance
Regulatory bodies, such as the FDA, MHRA, and EMA, mandate cleanroom validation for industries like pharmaceuticals, medical devices, and biotechnology. By adhering to these regulations, you demonstrate your commitment to safety, efficacy, and compliance.

Operational Efficiency
Cleanroom validation identifies areas of improvement within your facility, allowing you to optimize your processes and minimize operational disruptions. By addressing potential issues proactively, you can enhance productivity, reduce downtime, and maximize resource utilization.

Employee Safety
Cleanrooms often handle hazardous materials, and maintaining a safe working environment is crucial for your employees. Regular validation provides assurance that appropriate control measures are in place to protect personnel from airborne contaminants and other occupational hazards.

Alpha Linear performs cleanroom validation as per ISO 14644-1
This test measures the concentration and size distribution of airborne particles in the cleanroom. It determines compliance with specified cleanliness classes, ensuring that the air quality meets the required standards.
By measuring and evaluating the airflow patterns, this test ensures proper air circulation within the cleanroom. It verifies that airflows are directed appropriately to prevent the buildup and spread of contaminants.
This test assesses the pressure differentials between adjacent areas within the cleanroom. It ensures that air flows from clean to less clean areas, minimizing the risk of cross-contamination.
The filter integrity test confirms the effectiveness of high-efficiency particulate air (HEPA) filters. It checks for leaks and ensures that the filters are performing optimally in removing contaminants from the air.
This test assesses the cleanroom’s ability to return to its desired cleanliness level after a disturbance. It verifies the efficiency of the cleanroom’s recovery mechanisms, allowing you to assess its resilience to potential disruptions.
This test evaluates the integrity of the cleanroom’s physical barriers, such as walls, ceilings, doors, and windows. It ensures that there are no leaks or breaches that could allow contaminants to enter or escape the cleanroom.
At Alpha Linear, we understand the criticality of cleanroom validation for your operations. Our team of experienced professionals is equipped with the latest technology and expertise to perform these tests accurately, efficiently, and in compliance with regulatory requirements. With our comprehensive validation services, you can have peace of mind knowing that your cleanroom maintains the highest standards of cleanliness and integrity.
Contact us today to schedule your cleanroom validation and ensure the success and compliance of your operations. Alpha Linear, your dedicated partner in cleanroom validation.